Nuvaxovid
Novavax Announces Shipments Of Its. This will enable us to start offering the Nuvaxovid.
News European Commission Grants Conditional Marketing Authorisation For Novavax S Covid 19 Vaccine Nuvaxovid Paul Ehrlich Institut
This type of vaccine contains part of the coronavirus spike protein.

. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria.
Nuvaxovid is packaged as a ready-to-use liquid formulation in a vial containing ten doses. Most important facts about the new vaccine Nuvaxovid. Beslutet är temporärt och gäller från.
The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. A booster dose of Nuvaxovid may be given to people aged 18 years and. WHO lists 10th COVID-19 vaccine for emergency use.
Nuvaxovid contains a version of a protein found on the. 1 day agoThe US company Novavax came up with another vaccine to fight the virus - Nuvaxovid. This vaccine is currently being used in Sweden and as of date a total of 7000.
Novavax Nuvaxovid COVID-19 vaccine. A full list of ingredients for the qualitative and quantitative. The use of this vaccine should be in accordance with.
Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. The MHRA can confirm that Nuvaxovid does not contain any components of animal origin. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes.
Cambridge Mass and Osaka Japan April 19 2022 Takeda today announced that it has received manufacturing and marketing approval from the Japan Ministry of Health. Nuvaxovid is given as two injections usually into the muscle of the upper arm 3 weeks apart. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix. The first batch of Nuvaxovid is expected to arrive in. Novavaxin Nuvaxovid-koronarokote antaa suojaa SARS-CoV-2 viruksen aiheuttamaa infektiota ja COVID.
Nuvaxovid is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in individuals 12 years of age and older. Age 18 and older. The Swedish Public Health Agency is pauses Covid-19 vaccinations using the Nuvaxovid vaccine for people under the age of 30.
The flu vaccine and the hepatitis B vaccine which. Approved by Health Canada. Unlike mRNA vaccines such as Pfizer and Moderna the Novavax vaccine uses a longer-standing protein-based technology.
2 days agoPublicerad idag 0702. About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
Nuvaxovid contains a version of a protein found on the. The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its. Det eftersom att data från.
Your immune system cells recognise the spike protein as a threat and begin building an immune response. Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older.
Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022

Eu Regulator Backs Use Of Novavax Covid Shot As A Booster
Switzerland S Federal Office Of Public Health Recommended Nuvaxovid Nvx Cov2373 As A Heterologous And Homologous Booster For Immunization To Prevent Covid 19 Caused By Severe Acute Respiratory Syndrome Novavax
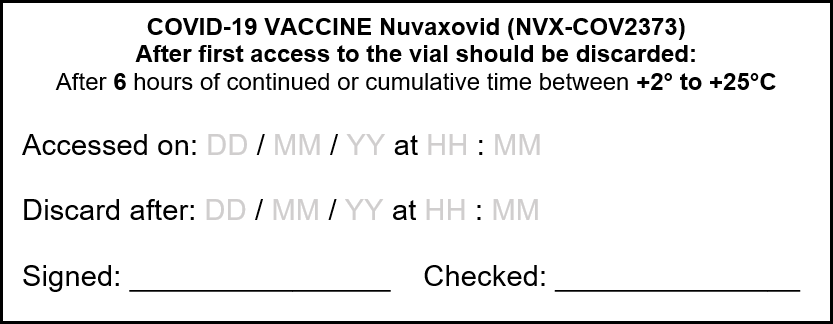
Management Of Covid 19 Vaccine Nuvaxovid Novavax From Refrigerator To Administration Vaccination

Cdc Endorses Novavax Covid Shot For Adults Fortune
Fda Authorizes Novavax Covid 19 Vaccine For Emergency Use In Us Abc News
Ema Chmp Recommends Cma For Novavax S Covid 19 Vaccine As Booster

Nuvaxovid Fifth Vaccine Against Covid Authorised In Eu Euractiv Com
Novavax Nuvaxovid Gets Expanded Conditional Marketing Authorization In Eu For Use As Booster For Adults Aged

Novavax Covid 19 Vaccine To Be Rolled Out In Australia From Next Month

Nuvaxovid Covovax Novavax Vaccine Covid 19 Info Vaccines

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl

Novavax Says Initial 1m Doses Of Nuvaxovid Covid 19 Vaccine Are Available In Uk

European Union Authorizes Novavax Booster

Vaccine Against Coronavirus Nuvaxovid Novavax Niph

Covid 19 Vaccination About The Nuvaxovid Novavax Vaccine Youtube
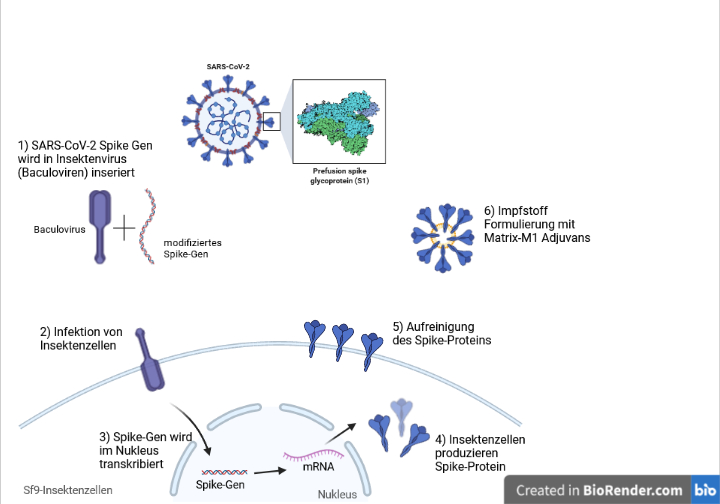
Nuvaxovid The New Subunit Sars Cov 2 Vaccine Mci Innsbruck
Allegheny County Health Department Will Start Administering Novavax Vaccine Pittsburgh Post Gazette